Views: 0 Author: Site Editor Publish Time: 2025-06-07 Origin: Site
The energy band gap is the smallest energy needed. It helps an electron move from a low to a high energy state. This is very important for solar cells. It decides how well they take in sunlight and turn it into power. For instance, a test model with special materials absorbed 80% of sunlight. It also reached 190% efficiency, going beyond normal limits. Learning about the energy band gap can help make solar cells better. This can lead to new ideas in clean energy.

The energy band gap is the smallest energy needed for electrons to move and make electricity in solar cells.
Picking materials with a band gap near 1.5 eV helps solar cells take in sunlight better and waste less energy.
Each material has its own band gap, which changes how well it turns sunlight into electricity.
Special designs like multi-junction solar cells use layers with different band gaps to catch more sunlight and work better.
Improving the band gap can cut costs and lead to new solar ideas, making clean energy easier to get.
Perovskite materials are very promising because they are efficient and work well even in dim light.
Studying band gaps is important for making solar technology better and helping the world use clean energy.
Knowing about the energy band gap helps people choose the best solar panels for their needs.
The energy band gap is a key idea in semiconductors. It shows the least energy needed for an electron to move. Electrons jump from the valence band, where they stay with atoms, to the conduction band, where they move freely. This jump is needed to make electricity in solar cells.
Think of the band gap as a barrier for electrons. Electrons need enough energy to cross it. Without enough energy, they stay stuck and cannot help make electricity.
In semiconductors, the band gap controls how electrons react to sunlight. When sunlight hits a solar cell, photons (light particles) give energy to electrons. If the photon’s energy matches or beats the band gap, electrons absorb it and jump to the conduction band. This jump creates electricity, which powers devices.
But not all photons help in this process. For example:
Photons with less energy than the band gap pass through without being absorbed.
Photons with energy equal to the band gap are absorbed well and help make electricity.
Photons with more energy than the band gap lose extra energy as heat, wasting it.
This shows why choosing the right materials with the best band gap is important for solar cells.

The band gap is vital for turning sunlight into electricity. When sunlight hits a solar cell, photons meet the semiconductor material. If the photon’s energy matches the band gap, electrons absorb it and move to the conduction band. This movement creates electric current, which powers devices.
New technologies like Intermediate Band Solar Cells (IBSCs) improve this process. These cells add extra energy levels in the band gap. They absorb photons with lower energy, using more sunlight. This can raise efficiency to 63.2%, much higher than usual.
Photon energy and the band gap decide how well a solar cell works. Materials with a band gap of about 1.5 eV are great for solar cells. This value balances sunlight absorption and reduces heat loss.
The table below shows how materials with different band gaps perform:
| Material Type | Cutoff Wavelength (nm) | Efficiency (%) |
|---|---|---|
| BaZrS3 | 725 | 18.13 |
| (Ba,Ca)ZrS3 | 983 | 22.23 |
| Ba(Zr,Sn)S3 | 837 | 21.84 |
| BaZr(S,Se)3 | 918 | 22.71 |
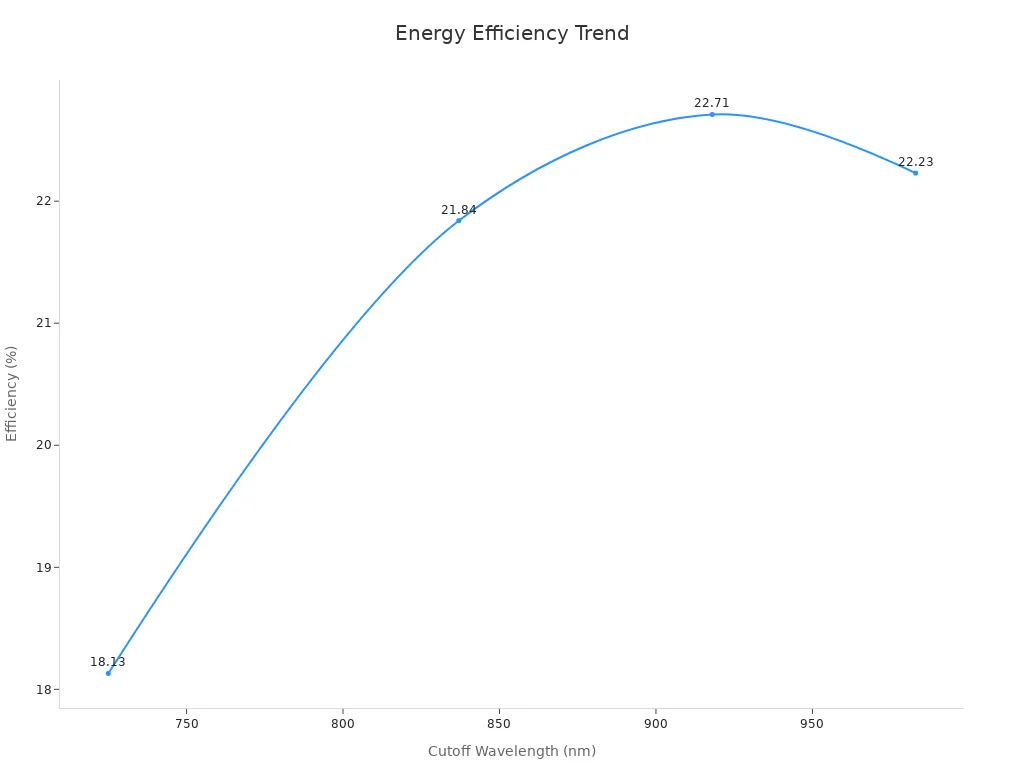
This table shows how the band gap affects solar cell efficiency. Materials with band gaps close to sunlight energy work better. They convert more sunlight into electricity, making them more useful.
A band gap of 1.5 eV is great for solar cells. It balances sunlight absorption and energy loss. Materials with this band gap absorb many sunlight wavelengths. This helps make more electricity.
Studies show band gaps between 1.04 eV and 1.69 eV affect efficiency. For example:
| Band Gap (eV) | Efficiency Effect | Notes |
|---|---|---|
| 1.04 - 1.69 | Changes with band gap | Best band gap is 1.21 eV; higher gaps lower absorption and current. |
This shows materials near 1.5 eV work better for making electricity.
The right band gap balances sunlight absorption and heat loss. A low band gap absorbs less sunlight, lowering efficiency. A high band gap wastes energy as heat.
For example, perovskite solar cells with a graded band gap are efficient. They reach 22.35% power conversion efficiency. They also have a short-circuit current of 24.57 mA/cm² and voltage of 1.07 V. This shows how the right band gap improves energy use and reduces heat loss.
The band gap controls how solar cells absorb light and make power. When the band gap matches sunlight energy, electrons move and create electricity.
Different band gaps change performance. For example:
A high band gap needs thicker materials to keep current steady.
A low band gap absorbs less light, lowering power.
Solar cells work better when the band gap matches sunlight.
Materials with different band gaps show how efficiency changes. For example:
Thicker materials increase short-circuit current and power efficiency.
A top band gap of 1.7 eV and bottom band gap of 1.28 eV give 32.71% efficiency.
Studies confirm these results:
| Bandgap Energy (eV) | Efficiency Effect | Source |
|---|---|---|
| ~0.7 | Local max efficiency | Martí & Araujo, 1996 |
| ~1.0 | Global max efficiency | Wanlass et al., 2005 |
| Varies by spectrum | Flexible material choice | Bremner et al., 2008 |
These examples show how the right band gap improves light absorption and power, making solar cells better.

Silicon is the most common material in solar cells. Its band gap energy is about 1.1 eV. This makes it good at absorbing sunlight and making electricity. Silicon can capture a large part of sunlight, making it great for solar panels.
Silicon solar cells have reached impressive efficiency levels. For example:
The highest possible efficiency for silicon cells is 32.33%.
A thin 15 μm silicon film reached 31% efficiency with better design.
The best real-world silicon solar cell has 26.7% efficiency.
These results show silicon's ability to perform well in solar energy systems.
Silicon also has some downsides. Its band gap is not perfect for top efficiency. High-energy photons lose energy as heat when absorbed by silicon. Also, silicon’s indirect band gap needs thicker materials to absorb sunlight. This makes production more expensive.
New materials are being developed to solve these issues. They aim to absorb light better and improve efficiency.
Perovskite materials are becoming popular for their high efficiency. Their band gap ranges from 1.5 eV to 2.3 eV. This range is great for absorbing sunlight and making electricity. Scientists are working to reduce energy losses in perovskite cells. By keeping electrons longer, they have improved efficiency.
Perovskite materials also work well in tandem solar cells. These combine perovskites with other materials for better results. Indoors, perovskite solar cells have reached nearly 45% efficiency. This makes them useful for powering small devices in low light.
Other materials like cadmium telluride (CdTe) and gallium arsenide (GaAs) also have benefits. CdTe has a band gap of about 1.45 eV, close to the best value for solar cells. It absorbs light well and is affordable. GaAs, with a band gap of 1.43 eV, is very efficient. It often reaches over 30% efficiency in labs.
The table below shows band gap energy for different materials:
| Material | Band Gap (eV) | DFT Approximation Used |
|---|---|---|
| Cs2AgSbCl6 | 1.163 | HSE06 |
| Cs2AgSbBr6 | 0.850 | HSE06 |
| Cs2AgSbI6 | 0.305 | HSE06 |
| Rb2CuSbCl6 | 1.140 | DFT calculations |
| K2CuSbCl6 | 1.123 | DFT calculations |
| Cs2AgBiBr6 | 1.93 | GGA-PBE |
| Cs2GeSnCl6 | 1.798 | GGA |
| Cs2GeSnBr6 | 1.044 | GGA |
| Cs2GeSnI6 | 0.474 | GGA |
| Cs2BBiX6 | 1.00 - 1.75 | Various DFT approaches |
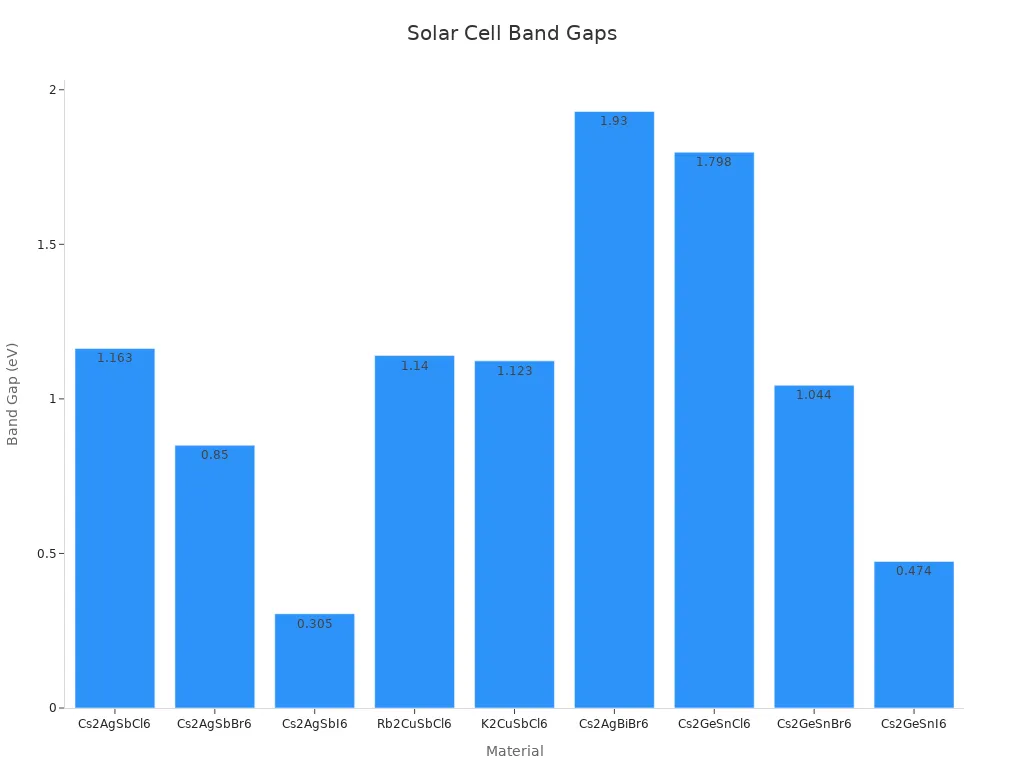
This table shows the variety of materials for solar cells. Each material has unique features to improve efficiency and performance.
The band gap of solar cells can be changed by altering materials. Adding small amounts of other atoms, called doping, changes a material's properties. For example, adding chromium to titanium dioxide (TiO₂) lowers its band gap from 3.40 eV to 2.70 eV. This helps it absorb sunlight better. Mixing iron pyrite with ruthenium also improves its performance by changing its band gap.
These methods help scientists match the band gap to sunlight energy. This makes solar cells absorb more light and work better. Tools like scanning Kelvin probe microscopy make this process more accurate. These tools measure things like voltage and energy depth. This helps improve the band gap for better results.
Some solar cells use layers with different band gaps. These are called multi-junction solar cells. Each layer absorbs a different type of sunlight. The top layer catches high-energy light, while the bottom layer absorbs lower-energy light.
This design makes solar cells much more efficient. Some multi-junction cells have over 40% efficiency. Combining materials like perovskites and silicon creates tandem cells. These cells work well in different lighting, making them useful in many places.
Improving the band gap makes solar cells more efficient. When the band gap matches sunlight energy, more light is absorbed. This creates more electricity. Perovskite solar cells now reach 26.49% efficiency, a big improvement.
Optimized band gaps also help solar cells work in different lighting. For example, perovskite cells are great indoors. They reach nearly 45% efficiency in low light. This makes them useful for homes and small devices.
Better band gaps not only improve efficiency but also cut costs. Optimized materials need to be thinner, which lowers production costs. Methods like doping and multi-layer designs make solar cells better without making them harder to produce.
Improving band gaps inspires new solar technologies. Scientists are testing new materials and designs to make solar cells even better. These advances make solar energy cheaper and more sustainable, helping the world use cleaner energy.
Improving band gaps faces big problems like material stability. Some advanced materials break down after long sunlight exposure. This makes them less reliable for solar cells. Making these materials in large amounts is also hard. It needs careful control, which is tough to do. For example, perovskite materials work well but don’t last long. This stops them from being widely used.
Another problem comes from mixing many elements in materials. More elements can create unwanted compounds. This makes production harder and less predictable. Computer models help solve this, but they cost a lot and aren’t always accurate. The table below shows key points about these problems:
| Evidence Description | Key Points |
|---|---|
| Computational costs and inaccuracies in dopability modeling | High computational costs hinder the widespread use of advanced band gap materials. |
| Phase competition affecting dopability | Increased number of elements leads to more possible compounds, complicating the phase diagram. |
| Predictive accuracy of linear models compared to complex methods | Simple models can predict dopability ranges with similar accuracy to complex machine learning techniques. |
These problems show the need for new ideas to make materials more stable and easier to produce.
Making advanced materials for solar cells costs a lot of money. These materials often need rare elements and expensive methods. This raises the price of solar panels, making them harder to afford. Also, designing these materials is tricky. Multi-layer solar cells need different band gaps in each layer. This requires special manufacturing steps.
Researchers are working on ways to lower costs and simplify production. Using easier computer models can save money while staying accurate. These efforts aim to make solar panels cheaper and better for everyone.
Quantum dots are tiny particles that bring new ideas to band gap research. Changing their size lets you control how they absorb light. This helps solar cells turn sunlight into electricity more efficiently. Quantum dots shift energy levels, improving how electrons move. This boosts their ability to make power.
Recent studies show their potential. For example:
CuLaSe₂ quantum dots raised power efficiency by 13.2%.
Adding zinc to CuLaSe₂ improved circuit efficiency from 1.85% to 2.20%.
These examples show how quantum dots can make solar cells work better and be more flexible.
Hybrid materials mix different substances to improve solar cells. Perovskite hybrids, for example, save energy and cut costs. By 2050, perovskite cells could lower energy use by 30.66%. Silicon-based systems might only save 25.51%. Perovskites could also save $443.71 USD yearly, compared to $369.26 USD for silicon cells.
But hybrid materials have environmental issues. Perovskites release more CO₂ during production. This means it takes longer to balance their environmental impact—about 6.81 years. Still, their high efficiency and low cost make them important for future research.
Quantum dots and hybrid materials offer exciting possibilities. They aim to solve current problems and create better, greener solar cells.
The energy band gap is key to making solar cells efficient. Picking materials with the right band gap helps solar cells absorb sunlight. This sunlight is then turned into electricity, boosting energy output.
Recent progress shows why the band gap is important:
Perovskite solar cells now reach 26.1% efficiency, beating silicon cells.
Tandem solar cells use different band gaps to capture more sunlight. These cells can reach up to 40% efficiency.
Wide band gap perovskites work well indoors with artificial light.
In farming, wide band gap materials allow crops to grow while making energy.
These examples show how improving the band gap can make solar technology better and more useful.
The energy band gap is vital for clean energy's future. Better solar cells mean less need for fossil fuels and more clean energy use. Materials with good band gaps help solar panels work in many places, like cities or farms.
Wide band gap materials also create new possibilities. They improve solar panels in low-light areas, making solar energy available everywhere. As scientists improve band gap technology, solar energy will become cheaper and more common. This will speed up the switch to clean energy worldwide.
Band gap research is crucial for global energy plans. Better solar cells mean more electricity from the same sunlight. This lowers renewable energy costs and makes it compete with fossil fuels.
Wide band gap materials also help save energy in other ways. They are used in electronics to cut energy loss during power transfer. This helps build smarter energy grids and better renewable systems. As countries aim to cut carbon emissions, band gap improvements make clean energy more effective.
Band gap research helps more than just solar cells. Wide band gap materials are improving many energy technologies.
| Trend Description | Impact on Energy Technologies |
|---|---|
| Growing need for energy-saving devices | Wide band gap materials improve power electronics for better performance. |
| Rise of electric vehicles | These materials work well at high temperatures and voltages, helping EVs. |
| Expanding renewable energy systems | Wide band gap materials improve power generation and distribution systems. |
Materials like gallium nitride (GaN) and silicon carbide (SiC) are changing industries. For example:
Renewable energy uses these materials to improve power systems.
5G networks rely on them for faster and better communication.
These advancements show how band gap research improves solar energy and other fields, leading to a greener future.
The energy band gap is crucial for solar cells. It decides how well they turn sunlight into electricity. Improving the band gap boosts efficiency and sparks new ideas in solar technology. For example, special designs like the "Cliff" structure help reduce energy loss. This improves the open-circuit voltage (V_OC). On the other hand, the "Spike" structure blocks energy flow, lowering efficiency.
| Heterojunction Structure | Effect on Performance | Key Details |
|---|---|---|
| Cliff | Helpful | Cuts energy loss, raises open-circuit voltage (V_OC) |
| Spike | Harmful | Blocks energy flow, reducing overall efficiency |
More research is needed to solve problems and improve solar cells. This will help create cleaner energy for the future.
The energy band gap is the smallest energy needed for an electron to jump from a low energy level to a higher one. This jump is what helps solar cells make electricity.
The band gap decides how well a solar cell takes in sunlight and turns it into electricity. Picking the right band gap makes the cell work better and lose less energy.
The best band gap for solar cells is about 1.5 eV. This amount lets the cell absorb sunlight well and avoid wasting energy as heat.
Different materials have their own band gaps. For example, silicon’s band gap is 1.1 eV, while perovskites range from 1.5 to 2.3 eV. These differences change how much sunlight they can turn into electricity.
Yes, the band gap can be changed by adding other atoms to materials or stacking layers with different band gaps. These methods help solar cells take in more sunlight and work better.
If the band gap is too high, energy is wasted as heat. If it’s too low, the cell doesn’t absorb enough sunlight. Both problems make the solar cell less efficient.
Yes, materials like perovskites and gallium arsenide can work better than silicon. They have better band gaps and higher efficiency, but they might cost more or not last as long.
Improving the band gap helps solar cells make more electricity. This supports global plans to use less fossil fuel and switch to clean energy.
Tip: Knowing about the energy band gap can help you pick the best solar panels for your needs.